Abstract
Background: Historically, hemophilia A has been considered a male disease, leading to a common misconception that carriers are asymptomatic. Hemophilia A carriers have on average half the factor VIII (FVIII) activity (FVIII:C) observed in healthy individuals and they can experience excessive bleeding even with normal FVIII levels. However, bleeding symptoms in women/girls with hemophilia A and in symptomatic carriers are often underrecognized and undertreated. Furthermore, there are limited clinical data available on the management of bleeding in these patients, and improved, evidence-based treatment guidelines on the management of hemophilia A in women/girls are required.
Aims: To evaluate the efficacy and safety of the recombinant FVIII simoctocog alfa (Nuwiq®) in women/girls with hemophilia A undergoing major surgery.
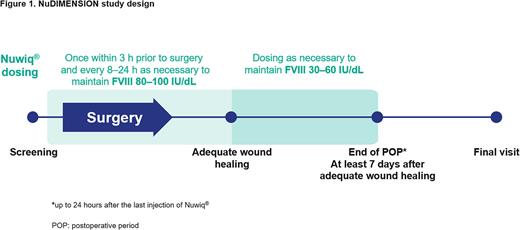
Methods: NuDIMENSION is a prospective, open-label, single-arm, multicenter study to be conducted at approximately 15 sites worldwide. Women/girls aged ≥12 years of age, diagnosed with moderate or mild hemophilia A (FVIII:C >1% and <40%), scheduled to undergo elective major surgery, and with no current or past FVIII inhibitors are eligible for the study. Simoctocog alfa will be administered once within 3 hours prior to surgery and repeated as necessary every 8-24 hours to achieve a FVIII plasma level of 80-100 international units (IU)/dL until adequate wound healing. Simoctocog alfa dosing will continue for at least another 7 days to maintain a FVIII plasma level of 30-60 IU/dL (Figure 1). The primary endpoint is the overall perioperative hemostatic efficacy ("success" or "failure") of simoctocog alfa in women/girls with hemophilia A undergoing major surgery. Hemostatic efficacy will be assessed at the end of surgery and at the end of the postoperative period (i.e., up to 24 hours after the last injection of simoctocog alfa), both using an objective 4-point ordinal hemostatic efficacy scale, and adjudicated by the Independent Data Monitoring Committee using a pre-defined algorithm. Secondary endpoints include assessment of intra- and postoperative hemostatic efficacy, perioperative FVIII plasma levels, and incidence of adverse events, including inhibitor formation. Target enrollment is 23 patients to document 26 major surgeries. Each subject can have multiple independent surgeries that will be counted as separate procedures. The study is planned to start in Q4 2022.
Conclusions: The results from the NuDIMENSION study will contribute to the evidence needed to generate specific treatment guidelines for the management of bleeding in women/girls with hemophilia A undergoing major surgery.
Disclosures
Oldenburg:Biotest: Consultancy, Honoraria, Other: travel, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Other: travel, Speakers Bureau; Biogen: Consultancy, Honoraria, Other: travel, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: travel, Research Funding, Speakers Bureau; Sobi: Consultancy, Honoraria, Other: travel, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Other: travel, Research Funding, Speakers Bureau; Chugai: Consultancy, Honoraria, Other: travel, Speakers Bureau; Biomarin: Consultancy, Honoraria, Other: travel, Speakers Bureau; CSL Behring: Consultancy, Honoraria, Other: travel, Research Funding, Speakers Bureau; Bayer: Consultancy, Honoraria, Other: travel, Research Funding, Speakers Bureau; Octapharma: Consultancy, Honoraria, Other: travel, Research Funding, Speakers Bureau; Freeline: Consultancy, Honoraria, Other: travel, Speakers Bureau; Grifols: Consultancy, Honoraria, Other: travel, Speakers Bureau; Spark Therapeutics: Consultancy, Honoraria, Other: travel, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Other: travel, Speakers Bureau. Marquardt:Baxalta: Honoraria; Chugai: Honoraria; Bayer: Consultancy, Honoraria; Takeda: Other: travel; Octapharma: Consultancy, Honoraria, Other: travel; Sobi: Consultancy, Honoraria, Other: travel; CSL Behring: Consultancy, Honoraria; Novo Nordisk: Consultancy, Honoraria, Other: travel; Pfizer: Consultancy, Honoraria, Research Funding; Roche: Honoraria. Jansen:Octapharma: Current Employment. Knaub:Octapharma AG: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal